What Happens if a Trach Comes Out
Note: this guideline is currently under review.
Introduction
Aim
Definition of Terms
Related Documents
Tracheostomy Kit
Emergency Management
The Resuscitation Flowchart (under review)
Complications
Post-Operative management of a new tracheostomy
Routine tracheostomy management
- Equipment and environment
- Supervision and Monitoring
- Leaving the ward
- Humidification
- Suctioning
- Abnormal Secretions
- Tracheostomy Tie Changes
- Tracheostomy Tube Changes
- Stoma Care
- Feeding and Nutrition
- Oral Care
- Communication
- Discharge Planning
- Tracheostomy Decannulation
Documentation
Special Considerations
Companion Documents
Evidence Table
References
Introduction
A tracheostomy is a surgical opening into the trachea below the larynx through which an indwelling tube is placed to overcome upper airway obstruction, facilitate mechanical ventilator support and/or the removal of tracheo-bronchial secretions.
Aim
The aim of the guideline is to outline the principles of management for patients with a new or existing tracheostomy for clinicians at the Royal Children's Hospital (RCH).
Definition of terms
- Decannulation: removal of a tracheostomy tub
- Heat moisture exchangers (HME): a hygroscopic material that retains the child's exhaled heat and moisture, which is then returned to subsequent inhaled air (gas).
- Humidification: the mechanical process of increasing the water vapour content of an inspired gas.
- Neopuff® : is a flow controlled, pressure limited mechanical device specifically designed for neonatal resuscitation. Breaths are delivered by occluding a T piece. Peek Inspiratory Pressure (PIP) is pre-set, and PEEP can be adjusted using the valve on the T piece. NeopuffTM is the resuscitation device used at the bedside in Neonatal Unit at RCH
- Stoma: a permanent opening between the surface of the body, and an underlying organ (in this case, between the trachea and the anterior surface of the neck).
- Tracheostomy: a surgical opening between 2 - 3 ( or 3 - 4) tracheal rings into the trachea below the larynx
- Tracheal Suctioning: is a means of clearing the airway of secretions or mucus through the application of negative pressure via a suction catheter.
- Trache or Tracheostomy tube: a curved hollow tube of rubber or plastic inserted into the trachea to relieve airway obstruction, facilitate mechanical ventilation or the removal of tracheal secretions. There are a variety of different tracheostomy tubes available.
Related Documents
- Aseptic technique
- Emergency Procedures
Tracheostomy Kit
A tracheostomy kit is to accompany the patient at all times and this must be checked each shift by the nurse caring for the patient to ensure all equipment is available.
A key concept of tracheostomy management is to ensure patency of the airway (tracheostomy tube). A blocked or partially blocked tracheostomy tube may cause severe breathing difficulties and this is a medical emergency. Immediate access to the tracheostomy kit (equipment) for the individual patient is essential.
Tracheostomy kit contains
- One tracheostomy tube of thesame size insitu (with introducer if applicable)
- One tracheostomy tubeone size smaller (with introducer if applicable)
- Spare inner tubes for double lumen trache tubes (if applicable)
- Spare ties (cotton and/or Velcro)
- Scissors
- Resuscitation bag and mask (appropriate size for patient)
- One way valve(community use only)
- Wall or portable suction equipment
- Appropriate size suction catheters
- 0.9% sodium chloride ampoule and 1ml syringe
- One Heat Moisture Exchanger filter (HME) or tracheostomy bib
- Fenestrated gauze dressing
- Cotton wool applicator sticks
- Water based lubricant for tube changes
- Mucous trap with suction catheter for emergency suction
- Occlusive tape (i.e. sleek)
- 10 ml syringe if cuffed tube insitu
Special safety considerations
- Ensure access to a working and charged phone and/or mobile phone at all times
- It is recommended that all patients have continuous pulse oximetry (SpO2) during all periods of sleep (day and night)and when out of line of sight of competent caregiver
- All children 6 years and under are to have cotton ties only to secure the tracheostomy tube.
- Children 6 years and over who are considered at risk of undoing Velcro ties should have cotton ties
- For patients with a newly established tracheostomy it is recommended that tracheal dilators are available at the patient's bedside until after the first successful tube change
- An information sheet that provides specific data regarding the date of last tracheostomy tube change, type and size of tracheotomy tube, (including inner diameter, outer diameter, length cuffed or uncuffed tube, cuff inflation, suctioning distance, critical alert if applicable), should be placed above each patient's bed (see Suctioning)
Emergency Management
The majority of children with a tracheostomy are dependent on the tube as their primary airway.
Cardiorespiratory arrest most commonly results from tracheostomy obstructions or accidental dislodgement of the tracheostomy tube from the airway.
Obstruction may be due to thick secretions, mucous plug, blood clot, foreign body, or kinking or dislodgement of the tube.
Early warning signs of obstruction include tachypnoea, increased work of breathing, abnormal breath sounds, tachycardia and a decrease in SpO2 levels.
Late signs of obstruction include cyanosis, bradycardia and apnoea - do not wait for these to develop before intervening.
The Resuscitation Flowchart
(currently under review, new chart coming soon)
For a tracheostomy patient follows APLS principles.
It is recommended that a copy of this flow chart is readily available e.g. placed in a prominent position at the bedside or in the patients bed chart folder.
Download the flowchart (PDF 21 KB)
Complications
Complications can be classified by timing: intraoperative; early (usually defined as the first postoperative week); late; and post-decannulation.
Complications in the first post-tracheostomy week include:
- Blocked tube (occluded cannula / mucous plugging)
- Bleeding from the airway/tracheostomy tube
- Stomal erosion
- Infection or cellulitis at the stoma site
- Air leak including Pneumothorax, pneumo-mediastinum or subcutaneous emphysema
- Respiratory and/or cardiovascular collapse
- Dislodged tube or accidental decannulation
- Granulation tissue in the trachea or at the stoma site
- Tracheo-oesophageal fistula
Late complications include:
- Acute airway obstruction
- Blocked tube (occluded cannula or mucous plugging)
- Infection (localised to stoma or tracheo-bronchial)
- Aspiration
- Tracheal trauma
- Dislodged tube
- Stomal or tracheal granulation tissue
- Tracheal stenosis
Post operative management of a new tracheostomy
After a tracheostomy is inserted, the patient is managed in either the Paediatric Intensive Care (PICU - Rosella) or Neonatal Unit (NNU - Butterfly) in the initial post-operative period and until after the first routine tracheostomy change.
- Ensure the tracheostomy equipment kit is present at the bedside with the patient.
- Patients return from theatre with stay sutures (nylon sutures) inserted on either side of the tracheal opening. The stay sutures are taped to the chest and labelled left and right. Pulling the stay sutures up and out will apply traction to the stoma opening to assist with insertion of the replacement tube.
- The stay sutures should remain in situ and securely attached to the chest wall until the first or second successful tube change.
- Trache stoma maturation takes approximately 5 – 7 days after insertion of the tracheostomy tube or 2 – 3 days if stoma maturation sutures are placed.
- The ENT team, in consultation with the parent medical team, will perform the first tube change, including the removal of the stay sutures.
- It is imperative that the first tracheostomy tie change is dealt with in the same manner as the first tracheostomy tube change with both nursing and medical staff present who are competent in tracheostomy management.
- The tracheal stoma in the immediate post-operative period requires regular assessment and wound management including once daily dressing change following cleaning of the stoma area or more frequently if required.
- The comfort of the patient is imperative throughout the post-operative period. Pain should be managed effectively as per RCH procedural pain management policy.
- Each child requires a Tracheostomy Tube Management Form to be completed and placed at the bedside. (see attached form)
Note:Most children will undergo their first tracheostomy tube change while in the intensive care environment. However, on occasions, following consultation between members of the PICU, ENT team and the parent unit, children may be transferred to a ward from PICU prior to their first tracheostomy tube change if they meet the following criteria:
- Have a non-critical airway i.e. these children are able to breathe and maintain their airway in the event of accidental decannulation.
- Are not dependent on or require positive pressure ventilation/CPAP via the tracheostomy.
Routine Tracheostomy Management
Routine tracheostomy management consists of:
- Equipment & environment
- Supervision and monitoring
- Humidification
- Suctioning
- Management of abnormal secretions
- Tracheostomy tube tie changes
- Tracheostomy tube changes
- Stoma care
- Feeding and nutrition
- Oral care
- Communication
- Transition to the community and discharge planning
- Tracheostomy decanulation
Equipment and environment
Each shift ensure
- All equipment for tracheostomy care is at the bedside and within easy access/reach
- Tracheostomy kit to be available with the patient at all times
- Suction equipment is set up with correct pressures (add link to suction procedure)
- Emergency oxygen equipment is set up and in working order
- Appropriate monitoring equipment available and correct alarm parameters set (as per Victor)
Supervision and monitoring
In determining the level of supervision and monitoring which is required, it is recommended each patient with a tracheostomy is assessed on an individual basis by the treating medical and nursing team4 taking into consideration the following factors:
- Age specific alarm limits (as per VICTOR chart)
- Clinical state
- Nature of the airway problem
- Ability to breathe and maintain their airway in the event of accidental decannulation
- Ability to clear own secretions
- Frequency of suction/tracheostomy tube interventions required
- Ventilation or respiratory support requirements e.g. CPAP, oxygen therapy
- Cognitive ability (neurological and age related)
Decisions regarding required level of supervision, clinical observations and monitoring are to be documented clearly in the patient's medical record by the treating medical/nursing team.
Monitoringmayinclude:
- Heart rate +/- continuous cardiac monitoring
- Respiratory rate
- Pulse oximetry continuous/overnight
- Oxygen requirements
- Work of breathing
- Temperature
- Blood pressure
- Behaviour - alert, irritable, lethargic
- Additional monitoring and/or assessment:Blood gases, tcCO2 and etCO2 as per medical orders.
It is recommended that all patients have continuous pulse oximetry (SpO2) during all periods of sleep (day and night) and when out of line of sight.
Children with a tracheostomy tube should be closely supervised when bathing or showering. They should also wear a HME filter or tracheostomy bib filter (unless on CPAP or ventilation) to minimise the risk of aspiration.
Leaving the ward
The patient's access to ward leave is assessed according to:
- Patient's clinical stability, clinical vulnerability.
- Caregiver competency in tracheostomy care – including knowledge and skill in airway (tracheostomy) emergency management.
- Ensure the tracheostomy kit accompanies the patient at all times
Humidification
A tracheostomy tube bypasses the upper airway and therefore prevents the normal humidification and filtration of inhaled air via the upper airway. Unless air inhaled via the tracheostomy tube is humidified, the epithelium of the trachea and bronchi will become dry, increasing the potential for tube blockage. Tracheal humidification can be provided by a heated humidifier or Heat and Moisture Exchanger (HME) or a Tracheostomy bib filter.
Heated humidification
Delivers gas at body temperature saturated with water which prevents the thickening of secretions. The temperature is set at 37°C delivering a temperature ranging from 36.5°C - 37.5°C at the tracheostomy site. Heated humidification for tracheostomy patients should be delivered via a humidifier as per the Oxygen clinical guideline (nursing). Indications for the use of heated humidification include:
- Oxygen delivery via tracheostomy mask
- Mechanical Ventilation
- Respiratory infection with increased secretions
- Management of thick secretions
Heat Moisture Exchanger (HME)
Contains a hygroscopic paper surface that absorbs the moisture in expired air. Upon inspiration the air passes over the hygroscopic paper surface and moistens and warms the air that passes into the airway.
- HME is recommended for all patients with a tracheostomy tube.
- HME fit directly onto the tracheostomy tube.
- Do not wet the HME filter prior to use
- HME are changed daily or as needed if the filter appears to be excessively moist or blocked.
- For small infants <10kg some HME filters may not be suitable. Consult Respiratory team to assess patient 's suitability
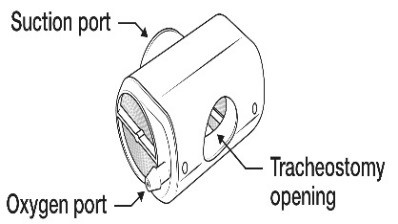
- HME with oxygen and suction port are suitable for low flow oxygen administration (as per oxygen guideline)
Tracheostomy bibs
Consist of a specialized foam that traps the moisture in the expired air, upon inspiration the foam moistens and warms the air that passes into the airway.
- At the RCH BuchananTM tracheostomy bibs are used.
- These are changed daily or more frequently as required
- Tracheostomy bibs are reusable - hand wash in warm water using a mild detergent/soap, then rinse thoroughly and allow to air dry.
- Tracheostomy bibs should be discarded monthly or more frequently if discoloured or the material is damaged.

Suctioning
Suctioning of the tracheostomy tube is necessary to remove mucus, maintain a patent airway, and avoid tracheostomy tube blockages. The frequency of suctioning varies and is based on individual patient assessment.
Indications for suctioning include:
- Audible or visual signs of secretions in the tube
- Signs of respiratory distress
- Suspicion of a blocked or partially blocked tube
- Inability by the child to clear the tube by coughing out the secretions
- Vomiting
- Desaturation on pulse oximetry
- Changes in ventilation pressures (in ventilated children)
- Request by the child for suction (older children)
Safety considerations:
- Tracheal damage may be caused by suctioning. This can be minimised by using the appropriate sized suction catheter, appropriate suction pressures and only suctioning within the tracheostomy tube.
- The depth of insertion of the suction catheter needs to be determined prior to suctioning. Using a spare tracheostomy tube of the same type and size and a suction catheter insert the suction catheter to measure the distance from the length of the tracheostomy tube 15mm connector to the end of the tracheostomy tube. Ensure the tip of the suction catheter remains with-in the tracheostomy tube.
- Record the required suction depth on the tape measure placed at the bedside and in the patient records. Attach the tape measure to the cot/bedside/suction machine for future use.
- Use pre - measured suction catheters (where available) to ensure accurate suction depth
- The pressure setting for tracheal suctioning is 80-120mmHg (10-16kpa). To avoid tracheal damage the suction pressure setting should not exceed 120mmHg/16kpa.
- It is recommended that the episode of suctioning (including passing the catheter and suctioning the tracheostomy tube) is completed within 5-10 seconds.
Equipment:
- Suction apparatus (wall attachment or portable unit)
- Suction canister
- Tubing
- Suction catheter
- Sterile water
Table 1: recommended suction catheter sizes
| Tracheostomy tube size (in mm) | 3.0mm | 3.5mm | 4.0mm | 4.5mm | 5.0mm | 6.0mm | 7.0 mm and > |
| Recommended suction catheter size (Fr) | 7 | 8 | 8 | 10 | 10 | 10 -12 | 12 |
Preparation
- Ensure Tracheostomy Kit is present
- Appropriate size suction catheters (with graduations if available)
- Tape measure with depth required for tracheostomy tube suctioning
- Appropriate suction pressure: correct suction pressure for use on a tracheostomy tube is80-120mmHg maximum when occluded. The Medigas suction gauges used on the wards are measured in kPa.The equivalent of 80- 120mmHg is 10-16kPa.
Procedure
- Explain to the patient and their family that you are going to suction the tracheostomy tube.
- Apply eye protection
- Perform hand hygiene, apply non-sterile gloves
- Remove HME, mask or circuit
- Peel open suction catheter end and attach to suction tubing, check and adjust suction pressure gauge to between 80 – 120 mmHg.
- Utilizing a non-touch technique gently introduce the suction catheter tip into the tracheostomy tube to the pre-measured depth.
- Apply finger to suction catheter hole & gently rotate the catheter while withdrawing.Each suction should not be any longer than 5-10 seconds.
- Assess the patient's respiratory rate, skin colour and/or oximetry reading to ensure the patient has not been compromised during the procedure.
- Repeat the suction as indicated by the patient's individual condition.
- Look at the secretions in the suction tubing - they should normally be clear or white and move easily through the tubing. Document changes from normal colour and consistency and notify the treating team if the secretions are abnormal colour or consistency.
- Rinse the suction catheter withsterile water decanted into container (not directly from bottle).
- Replace suction catheter into the packaging
- Dispose of waste, remove gloves and perform hand hygiene
Note:
- Suction catheters are to be routinely replaced every 24 hours or at any time if contaminated or blocked by secretions.
- Suction water/and the container to be replaced every 24 hours.
- Routine use of 0.9% sodium chloride is not recommended as there is little clinical evidence to support this. However, in situations where this may be of benefit e.g., thick secretions and/or to stimulate a cough 0.5ml of 0.9% sodium chloride can be instilled into the tracheostomy tube immediately prior to the suction procedure.
Special safety considerations
Some patients may require assisted ventilation before and after suctioning. If required, this will be requested by the parent medical team or Respiratory CNC.
If the correct size suction catheter does not pass easily into the tracheostomy tube, suspect a blocked or partially blocked tube and prepare for immediate tracheostomy tube change.
Management of abnormal secretions
Changes in secretions e.g. blood stained or yellow and green secretions may indicate infection and or trauma of the airway.
Notify the parent team for review who may request sending a sputum specimen for culture and sensitivity and consider commencement of antibiotics.
Persistent blood stained secretions from the tracheostomy tube need to be investigated to determine the cause.
Tracheostomy tie changes
- If tie changes are required before the first tube change – it is imperative that the procedure must be undertaken with both medical and nursing staff present who are able to reinsert the tracheostomy tube in case of accidental decannulation and the appropriate equipment is available at the bedside.
- Tracheostomy tie changes are performed daily in conjunction with stoma care, or as required if they become wet or soiled to maintain skin integrity.
- It is preferable to secure new ties before removing the old ties
- As there is a potential risk for tracheostomy tube dislodgment when attending to tie changes aminimum of two people who are competent in tracheostomy care are required to undertake tracheostomy tie changes.
- During the tracheostomy tie change, if the old ties are removed prior to securing the new ties, one person is to maintain the airway by securing the tracheostomy tube in place and not removing the hand until the new tracheostomy ties are secured. The other person inserts the new ties into the flange and secures around the child's neck.
- If the ties become loose it is a priority to re-secure immediately.
- All Children 6 years and under are to have cotton ties only to secure the tracheostomy tube.
- Children 6 years and overwho are considered at risk of undoing Velcro ties should have cotton ties.
Equipment
- Tracheostomy kit
- Two equal lengths of cotton ties (approximately 40cm) or
- Velcro ties (for patients older than 6 years)
Procedure for changing cotton ties
- Explain to the patient and their family that you are going to change the tracheostomy ties.
- Apply eye protection
- Perform hand hygiene, apply non-sterile gloves
- Prepare two equal lengths of ties long enough to go around the child's neck.
- Position the patient; an infant or child may lie down with the neck gently extended by a small rolled towel placed under the child's shoulders. An older child may like to sit up in a bed or chair
- Insert a clean tie into the holes on each side of the flange
- On each side tie a single loop approximately 0.5cm from the flange on the tracheostomy tube.
- Then tie both sides together in a bow to secure.
- Check the tension of the ties.
- Allow one finger to fit snugly between the skin and the ties.
- Re-tie into in a double (reef) knot to secure.
- Cut off excess length of ties leaving approximately 3cm.
- Using scissors remove old ties and recheck tension of new ties.
- Dispose of waste, remove gloves, and perform hand hygiene.
- Observe around the patient's neck to check skin integrity.
NB: The old ties are to remain insitu until the clean ties are secured. In the event of removing existing ties prior to securing the tube with clean ties it is recommended a second person is present to hold the tracheostomy tube ensuring it remains in place until the ties are secured.
Procedure for changing Velcro ties
- Changing Velcro ties is a two person procedure.
- Check the Velcro on the tracheostomy ties prior to each use to ensure adhesiveness. If not adherent discard and replace.
- Apply eye protection
- Perform hand hygiene, apply non-sterile gloves
- One person holds the tracheostomy tube securely in place.
- The second person removes the existing Velcro ties and then inserts the clean Velcro ties through one side of the flange, passing the tie around the back of the patient's neck and inserting the Velcro tie through the other side of the flange.
- Adjust the ties to allow one finger to fit snugly between the skin and the ties.
- Check to ensure the Velcro is securely fastened
- Dispose of waste, remove gloves, and perform hand hygiene.
- Observe the patient's neck to check skin integrity.
- Wash Velcro ties daily in warm, soapy water, rinse and allow to dry completely before re-using.
Tracheostomy tube changes
The frequency of a tracheostomy tube changes is determined by the Respiratory and ENT teams except in anemergency situation. This can vary depending on the patient's individual needs and tracheostomy tube type.
It is imperative that thefirsttracheostomy tube change is performed with both nursing and medical staff who are competent in tracheostomy management are present and the tracheostomy kit is available at the bedside.
A minimum of two people who are competent in tracheostomy care are required for all tracheostomy tube changes (except in anemergencyif a second person is not readily available – e.g. transporting the child).
The tube change should occur before a meal or at least one-hour after to minimise the risk of aspiration.
The tube change procedure is performed using standard aseptic principles using a non-touch technique.
Equipment
- Tracheostomy Kit
- Suction device and appropriate sized suction catheters
- Small towel (rolled to place under the patient's shoulders to extend their neck)
- A cot sheet to wrap the patient (age dependant)
- Appropriate light/ illumination
Preparation
- Apply eye protection
- Perform hand hygiene, apply non-sterile gloves
- Prepare the equipment on a clean surface area
- Prepare new tracheostomy tube by removing it from the packaging/container, check the expiry dates and inspect for any signs of damage to the tube and then thread the ties into the flange and tie.
- Ensure the spare smaller sized tracheostomy tube is available within arm's reach
- If using Velcro ties insert the ties on one side of the flange only
- Clearly explain the procedure to the patient and their family/carer.
- Consider distraction techniques and or procedural sedation.
- Swaddle the patient if age appropriate by wrapping the arms and containing them in the sheet.
- Place the rolled towel under the patient's shoulders to extend their neck (unless contraindicated). The older child may find it more comfortable to sit upright with their head tilted back.
- Position the child so that you have good visibility and access to the stoma. If necessary extend the neck further and open the stoma wider by using your thumb and forefinger.
- Suction the existing tracheostomy tube immediately before removing the existing tube and inserting the new one.
- Dispose of waste, remove gloves, and perform hand hygiene.
Procedure
- Person 1 holds the existing tube with their hand and keeps secured in place
- Person 2 cuts and removes the cotton ties from around the child's neck. If using Velcro ties - undo and remove from the tracheostomy tube flange.
- Person 2 holding the new tube asks person 1 to remove existing tracheostomy tube
- Person 2 immediately inserts the new tube into the stoma and removes the introducer (if applicable).
- Person 2 holds the tube securely in place whilePerson 1 ties and secures the tracheostomy ties
- Person 1 checks the tension of the ties to allow that one finger will fit snugly/firmly between the skin and the ties, adjust if necessary. If using cotton ties, finish by making a double (reef) knot and cut off any excess fabric leaving approximately 3cm.
Observe the child immediately after the tube change to check they are breathing normally with no signs of respiratory distress and that air is moving in and out of the tube by:
- listening for sounds of air coming out of the tube
- looking at the rise and fall of the chest
- feeling with your hand for a flow of air
- Check the tube for blockages, damage and/or wear and tear
- Unless instructed otherwise, all tracheostomy tubes are a single use only item
- Single use tracheostomy tubes should be used once only and discarded after every tube change. Do not clean or re-use single use tubes.
- Clean reusable tracheostomy tubes, wash and dry reusable tubes according to the manufacturer's recommendations.
- Dispose of waste, remove gloves, and perform hand hygiene.
- Document procedure and device information in the patient medical record as per requirements stated below.
Note: If unable to reinsert tracheostomy tube follow emergency procedure.
Safety considerations
- A rare complication is for the tube to slip into a false passage instead of the airway. If there are any signs of breathing difficulties/respiratory distress remove the tube and reinsert (a new tube) via the stoma into the airway.
- Difficulties in re-inserting the tracheostomy tube can occur at any time. These occur usually as a result of one of the following:
- False tract
- Patient agitation or distress
- Closure of the stoma
- Spasm of the trachea
- Stoma is blocked by scar tissue (granuloma)
- Skin flaps
- Structural airway abnormalities e.g.: Tracheomalacia/Bronchomalacia or tracheal granulations
- At times the difficulty is for no obvious reason and cannot be explained
Stoma care
- Care of the stoma is commenced in the immediate post-operative period, and is ongoing.
- Inspect the stoma area at least daily to ensure the skin is clean and dry to maintain skin integrity and avoid breakdown
- Daily cleaning of the stoma is recommended using 0.9% sterile saline solution.
- After daily cleaning, ensure dressing inserted at stoma site
Equipment
- Tracheostomy kit
- Fenestrated gauze dressing
- 0.9% sodium chloride
- Cotton wool applicator sticks
Preparation
- Apply eye protection
- Perform hand hygiene, apply non-sterile gloves
- Collect and prepare all equipment for procedure on a clean surface area
Procedure
- Clearly explain the procedure to the patient and their family/carer
- Perform hand hygiene
- Use a standard aseptic technique using non-touch technique
- Position the patient. Infants and young children may lay on their back with a small rolled towel under the shoulders. An older child may prefer to sit up in a bed or chair.
- Perform hand hygiene and apply non-sterile gloves
- Remove fenestrated dressing from around stoma
- Inspect the stoma area around the tracheostomy tube
- Perform hand hygiene and apply non-sterile gloves
- Clean stoma with cotton wool applicator sticks moistened with 0.9% sodium chloride. Use each cotton wool applicator stick once onlytaking it from one side of the stoma opening to the other and then discard in waste.
- Continue cleaning stoma area as above with a new cotton wool applicator stick each time until the skin area is free of secretions, crusting and discharge.
- Allow skin to air dry or use a dry cotton wool applicator stick to dry.
- Insert the fenestrated gauze under the flanges (wings) of the tracheostomy tube to prevent chafing of the skin.
- Dispose of waste, remove gloves, and perform hand hygiene.
- Avoidusing any powders or creams on the skin around the stoma unless prescribed by a doctor or respiratory nurse consultants as powders or creams could cause further irritation.
Special considerations
- If signs of redness or excessive exudate present consider using a non-adhesive hydro cellular foam dressing e.g. Allevyn®.
- If visible signs of infection are present - discuss with parent medical team and consider obtaining a swab specimen for culture and sensitivity.
- If there are any signs of granulation tissue liaise with the Respiratory Nurse Consultants for appropriate management.
- The care of the stoma includes routine (minimum - daily) observation of the site and accurate documentation of the findings including the presence of any of the following:
- Redness
- Swelling
- Evidence of granulation tissue
- Exudate
- Increased discomfort or pain at the site
- Offensive odour
Refer to Respiratory Clinical Nurse Consultant for advice on the frequency and type of dressing required.
Feeding and nutrition
The tracheostomy tube may have an impact on the child's ability to swallow safely, therefore a swallowing evaluation by a speech pathologist is recommended prior to the commencement of oral intake. The speech pathologist may recommend the optimum method of feeding as well as the types and consistency of foods and liquids.
Consider a dietician referral to assess optimal nutritional intake – including oral versus tube feeding (PEG, PEJ or NG), continuous versus intermittent feeding.
Oral care
Patients with a tracheostomy have altered upper airway function and may have increased oral care requirements. Mouth care should assessed by the nurse caring for the patient and documented in the patient care record.
Communication
Children communicate in many different ways, such as using gestures, facial expressions and body postures, as well as vocalising. The tracheostomy may impact on the child's ability to produce a normal voice. For all patients with a new tracheostomy a referral to a speech pathologist for assessment and provision of communication aids is recommended.
Vocalisation depends on several factors such as
- Severity of airway obstruction
- Extent of vocal cord function
- The size and type of the tracheostomy tube insitu
- Respiratory muscle strength
- Cognitive ability and age related ability
Communication aides include
- Pen and paper
- Alphabet board
- Picture communication device
- Electronic devices - phone/tablets
- Teaching manual for Auslan signing
- One-way speaking valve attachment
For children with established tracheostomy tubes it is essential that the methods used for communication are identified via discussion with the patient (age appropriate), and the parent/primary caregivers. These methods should be documented in the medical record and verbally handed over to staff to ensure adequate communication and appropriate understanding of the patient and their needs.
One- way speaking valves
One-way speaking valves are a small plastic device with a silicone one-way valve, they sit on the end of the tracheostomy tube. The most commonly used at the Royal Children's are Passy-Muir™ one-way valves and the Tracoe™ modular valve.
The one-way valve opens on inspiration allowing air to enter the tracheostomy tube and closes on exhalation directing air up through the trachea, larynx and nose and mouth as in normal breathing and normal speech.
Not all children will be able to produce a vocal sounds or voice when the speaking valve is first used.
Various types of one-way speaking valves are available.
Benefits of using a one-way speaking valve include:
- Enhancing normal flow of air through the airway/nose and mouth
- Restoration of physiological PEEP
- Louder and clearer voice
- Improved ability to taste and smell food
- Improved secretion management
- Improved protection of the airways during swallowing and feeding
- Improves development of speech and babbling in infants/toddlers
Contraindications for one-way speaking valve assessment:
- Severe airway obstruction
- Vocal cord paralysis - adducted position
- Severe neurological deficit
- Tracheostomy tube with inflated cuff (any kind)
- Foam-filled cuff (even if deflated)
- Severe risk for aspiration
- Less than 7 days post-operative tracheostomy tube insertion
Before one-way speaking valve use:
One-way speaking valves are not suitable for all children with a tracheostomy. The child's tolerance to the one-way speaking valve will depend on their airway around and above the tracheostomy tube. To exhale sufficiently the child must have enough airway patency around the tracheostomy tube, up through the larynx and out of the nose and mouth. If exhalation is not adequate with the one-way speaking valve in place the child may become distressed and air trapping/breath stacking or barotrauma to the lungs may occur. Therefore, a joint assessment involving the Respiratory nurse consultant and a Speech pathologist is essential before the device is used to determine if the child has adequate airway patency.
To determine if the child has adequate airway patency consider:
- Diagnosis of severe laryngeal or tracheal stenosis/subglottic stenosis
- Size and type of the tracheostomy tube - appropriate to allow airflow through upper airway
- Nasal obstruction - e.g. nasogastric tubes/choanal atresia
Before using the one-way speaking valve ensure the child is:
- Medically stable
- Greater than 7 days post tracheostomy insertion
- Awake, alert and responsive
- Able to tolerate cuff deflation
- Doesn't have a foam cuffed tracheostomy tube insitu
- Has adequate patency of upper airway
- Does not have excessive tracheal secretions
- Able to manage their oral secretions
Contraindication to one-way speaking valve use:
- If you determine there is no or inadequate airway patency this is a contraindication to speaking valve use.
- If the child has prolonged excessive coughing and obvious discomfit with increased respiratory effort and air trapping - remove the valve immediately and reassess for adequate airway patency before a repeat trial.
- If airway patency adequate then aim to reassess the child at regular intervals to place the one-way speaking valve gradually increasing the time and frequency of use.
- one-way speaking valve may be contraindicated depending on type of cuffed tube e.g. foam cuff
Bedside assessment of airway patency and use of one-way speaking valve:
Preparation
- Apply eye protection
- Perform hand hygiene, apply non-sterile gloves
- Collect and prepare all equipment for procedure on a clean surface area
Procedure
- Explain procedure (age appropriate) to child and their family
- Suction the tracheostomy tube before the valve is attached and then as required.
- A cuffed tube must be fully deflated before attaching the speaking valve.
- Gently occlude tracheostomy tube with a gloved finger and observe for exhaled air from nose and mouth or vocalization.
- If finger occlusion is tolerated place the speaking valve on the end of the tracheostomy tube and observe for oral/nasal exhalation.
- If the one-way speaking valve is tolerated on the initial trial for a goal of 5 to 10 minutes.
- A management plan to gradually increase the length of time which the valve is used will be provided for the patient
- Once the child has adjusted to wearing the one-way speaking valve they should be able to wear it for long periods and be able to be wear at all awake periods, particularly during rehabilitative therapy sessions and when eating.
If the child fails to tolerate the one-way speaking valve:
- Remove the valve if any signs or symptoms of distress or changes in respiratory effort.
- As it can be more difficult for the child to exhale with the valve in place, the child may initially fail a trial of one-way speaking valve due to anxiety or discomfort. The child may need to slowly build up longer periods of one-way speaking valve use and placement will be repeated on subsequent days.
- Some children have difficulty adjusting to changes to their airways. Children may initially experience increased coughing due to restoration of a closed respiratory system, which re-establishes subglottic pressure and normalizes exhaled airflow in the oral/nasal chambers.
- In infants and young children consider using a device to secure the one-way speaking valve to the child's ties - to prevent accidental loss of the one-way speaking valve.
- Some speaking valves are suitable for use in combination with oxygen therapy and during ventilation.
Safety precautions when using one-way speaking valves:
- If the child has severe airway obstruction the speaking valve should not be used.
- In cuffed tracheostomy tubes - ensure cuff is completely deflated.
- The young child should always be supervised when wearing the speaking valve.
- The one-way speaking valve should not be worn when the child is sleeping.
- One-way speaking valves do not humidify the air - therefore may be unsuitable for children with copious thick secretions.
- If the one-way speaking valve is not functioning properly (i.e. sticking, noisy or vibrates) or the child shows signs of respiratory distress/discomfort, then remove the valve immediately and replace.
- Do not use in combination with HME (heat moisture exchanger)
- Ensure the one-way speaking valve is clean and not damaged in any way before each use.
- Discard and replace immediately if any signs of wear/tear or damage are noted.
- Remove valve before aerosol/nebulizer medication is administered
Care and cleaning of the valve:
- The one-way speaking valve should be cleaned at least daily after use by washing in warm mild soapy water, then rinsed thoroughly and allowed to air dry completely before reuse.
- Once dry and when not in use, it should be stored in an appropriate storage container
- Dispose of waste, remove gloves, and perform hand hygiene.
To avoid damage to the valve:
- do notwash in hot water
- do not use a brush on the valve
- do notuse alcohol, peroxide or bleach to clean the valve
Transition to the community and discharge planning
Referral to Complex Care Hub (CCH)
All children with a tracheostomy tube should be referred to Complex Care Hub after discussion with the family. The referral should be made as soon as possible following tracheostomy tube insertion to allow adequate time for the planning of in-home health care support prior to the patients discharge.
Following the referral a needs assessment will be undertaken by CCH team to determine the support required for the patient and their family.
- Internal referral to EPIC
- External referral form
The referring team is responsible for ensuring appropriate equipment for discharge is organised in collaboration with the Complex Care Hub or Equipment Distribution Centre.
This should occur in consultation with the ward nursing staff, respiratory nurse consultants and the parent collaboration with the Complex Care Hub or Equipment Distribution Centre.
Ensure all members of the medical, nursing and allied health teams are aware of the planned discharge date.
Education for primary care givers regarding tracheostomy care commences soon after insertion of the tube and is usually initiated by the respiratory CNC in collaboration with the parent unit nursing staff.
Principles of the care for children with a tracheostomy in the community who are supported by the Complex Care Hub are based on the recommendations of this clinical practice guideline and individualised care plans are developed specifically to the patient's care needs. These are located in the home care manuals provided by Complex care team.
Tracheostomy Decannulation
Decannulation is a planned intervention for the permanent removal of the tracheostomy tube once the underlying indication for the tracheostomy has been resolved or corrected
Assessment and decannulation management
- To formally assess whether the child can maintain their airway and ventilation adequately without the tracheostomy tube, an endoscopic/bronchoscopy is performed to evaluate if the underlying indication for the tracheostomy has been resolved, corrected, and to assess for other factors which might impede a successful decannulation for example: granulation tissue or supra-stomal collapse. This procedure is performed within 6 weeks prior to admission for decannulation.
- Following the endoscopic evaluation the ENT and Respiratory teams will determine and document in the patient record the child's specific decannulation plan.
Preparation
- Decannulation management is usually a staged process commenced as an outpatient clinic with assessment following capping of the tracheostomy tube. If this is tolerated it is continued at home with intermittent daytime/awake capping (using a decannulation cap) with caregiver supervision.
- Downsizing of the tracheostomy tube may be done in conjunction with the capping in order to assess how well the child manages with a smaller tracheostomy in their airway and to encourage the use of their upper airway.
- The decannulation process is performed in the hospital as an in-patient. This is usually a 3 – 4 day admission.
- The patient is nursed 1:1 for at least 8 hours post decannulation. At the end of this period the need for 1:1 nursing supervision of the patient is assessed by the patient's parent medical team. If complications with the decannulation are anticipated the patient should be nursed 1:1 for the first 24 hours post decannulation
Decannulation - Day 1
- The tracheostomy tube is downsized to a 3.5 mm tracheostomy tube or as according the patient specific decannulation management plan. Ensure documented plan for the decannulation process from the parent medical team
- Baseline observations including heart rate, respiratory rate, SpO2 (haemoglobin-oxygen saturation), and work of breathing are recorded.
- The tube is capped (occluded using a decannulation cap and the child is observed for any signs of increased respiratory effort or respiratory distress including:
- Tachypnoea
- Stridor
- Retraction
- Tachycardia
- Colour
- Decreased perfusion
- Oxygen desaturation or low oximetry reading
- Restlessness or anxiety
- Decreased cough effectiveness, swallow and voice quality
If the child is unable to tolerate the downsizing and capping of the tracheostomy tube a medical review is required as the trial of decannulation may not proceed and the tube may be upsized.
If the child tolerates downsizing and capping of the tube ensure patient vital signs remain within appropriate parameters for age & as per VICTOR chart. Additional monitoring: Overnight oximetry monitoring (downloadable) and sleep diary are recorded throughout the night.
The child is reviewed in the morning by the admitting team to determine whether the decannulation trial goes ahead or not.
Decannulation – Day 2
Decannulation is usually performed between the hours of 9am and 10am (following medical review).
Decannulationshould notbe performed unless a member of the parent medical team is present in the ward at the time of decannulation.Inform the ENT team of the planned decannulation prior to removal of the tracheostomy tube.
Note:Occasionally the trial of decannulation is unsuccessful requiring the need to re-insert the tracheostomy tube. This is an emergency procedure and it can occur at any time – ensure equipment is at bedside and remains with the child until the child is discharged.
Equipment
- Tracheostomy Kit
- Set of tracheostomy tubes (same size and smaller sizes than tube child has insitu down to a size 3mm – including additional size 3mm in freezer.
- Surgical scissors
- Tracheostomy ties or Velcro ties
- Suction equipment
- Gauze and an occlusive dressing – e.g. comfeel™ with hypafix borders or tegaderm™/opsite™ to cover the tracheostomy stoma
- Cotton wool applicators
- Small towel (if applicable)
- Oxygen equipment
- Manual Resuscitator bag
- Monitoring equipment
Preparation
- Apply eye protection
- Perform hand hygiene, apply non-sterile gloves
- Collect and prepare all equipment for procedure on a clean surface area
- Ensure the child has been fastedfor 2 hours priorto the decannulation (i.e. decannulation planned at 9am-10am fast from 7am)
- Obtain baseline observations including: heart rate, respiratory rate, SpO2 (haemoglobin-oxygen saturation), and work of breathing. Ensure patient vital signs are within appropriate parameters for age & as per VICTOR chart. Continue to visually observe and monitor patient continuously throughout the procedure
Procedure
- Clearly explain the procedure to the patient and their family/carer
- It is recommended that the child's caregiver/s are present during the decannulation procedure to alleviate the anxiety of the child.
- Perform hand hygiene
- Use a standard aseptic technique using non-touch technique
- Position the patient. Infants and young children may lay on their back with a small rolled towel under the shoulders. An older child may prefer to sit up in a bed or chair.
- Perform hand hygiene and apply non-sterile gloves
- Remove fenestrated dressing from around stoma
- Clean the stoma site and suction the tracheostomy tube immediately prior to decannulation
- Cut/undo tracheostomy tube ties
- Remove tracheostomy tube
- Observe closely for any signs of respiratory distress including:
- Tachypnoea
- Stridor
- Retraction
- Tachycardia
- Colour
- Decreased perfusion
- Oxygen desaturation or low oximetry reading
- Restlessness or anxiety
- Decreased cough effectiveness, swallow and voice quality
- Activity levels
- If no evidence of respiratory distress an occlusive dressing is applied to stoma site to ensure an airtight seal and reassess patient for any sign of respiratory distress.
Following decannulation:
Monitor the patient's vital signs - respiratory rate, heart rate, oxygen saturation, colour and work of breathing continuously throughout the procedure then observe and document:
- 15 minutely for the first hour
- Half hourly for the next 4 hours
- Hourly for 24 hours
- Continuous pulse oximetry (SpO2) during all periods of sleep (day and night) post decannulation for 24 hours.
- Observe carefully for any signs of airway obstruction or increased respiratory effort during sleep periods
- Call a MET for assistance as per RCH emergency guidelines
Immediately report any episodes of:
- Tachypnoea or bradypnoea
- Tachycardia or bradycardia
- SpO2desaturation
- Increased WOB – mild, moderate or severe - as evidenced by: sternal or intercostal retraction, tracheal tug, nasal flaring, or stridor
- Restlessness and or anxiety
- Colour change and or cyanosis
- Failure to clear secretions – gagging
- Offer light diet 2 hours after decannulation (unless contraindicated)
- Encourage the child to undertake their normal activities while on the ward.
- Avoid suctioning the stoma unless otherwise indicated in an emergency situation as this may cause trauma.
Note: The child is to remain on the ward for 24 hours post decannulation and should not leave the ward without medical approval and supervised by nursing staff competent in tracheostomy care.
Stoma site care post decannulation
- The stoma site is covered by a small gauze square and then by an occlusive dressing (sleek™/tegaderm™) until it has closed or no secretions are seeping out.
- Assess occlusive tracheal stoma dressing for air leaks every shift and document absence or presence of these air leaks in medical record.
- Stoma site to be assessed and cleaned and dressing applied daily or more frequently if indicated.
- Observe for skin reactions to dressing used – if redness or irritation trial alternative dressing
Decannulation - Day 3
Following the first 24 hours post decannulation:
- Patient may leave the ward if the parent team has assessed the patient to have a "safe airway"
- Encourage usual activities to assess exercise tolerance – if age appropriate consider exercise testing/respiratory function tests
- Encourage coughing to clear secretions from upper airway if required. If the child is not coughing and clearing secretions well, gentle oropharyngeal suction (only) may be performed. Contact the physiotherapist for support.
- Referral to speech pathology should be considered if the child does not resume normal voice production following decannulation or inadequate swallow.
Stoma site care post decannulation:
- The stoma site is covered by a small gauze square and then by an occlusive dressing (sleek™/tegaderm™) until it has closed or no secretions are seeping out.
- Assess occlusive tracheal stoma dressing for air leaks every shift and document absence or presence of these air leaks in medical record.
- Stoma site to be assessed and cleaned daily or more frequently if indicated.
- Observe for skin reactions to dressing used – if redness or irritation trial alternative dressing
Decannulation - Day 4
Discharge home
The child is usually discharged home when they're considered by the medical team to have a safe airway.
The average hospital length of stay post decannulation is 36 - 48 hours, however this maybe longer if clinically indicated.
Following a successful decannulation the family are able to return all tracheostomy and suctioning equipment on discharge from hospital but are encouraged to keep the pulse oximeter until seen at follow up outpatient appointment.
Advise the family/caregiver to observe for and contact the hospital and/or medical team ifany episodes of:
- Increased Work of Breathing as evidenced by: sternal/intercostal retraction, tracheal tug, nasal flaring, stridor
- Tachypnoea/bradypnoea
- SpO2 desaturation
- Restlessness/anxiety
- Colour change/ Cyanosis
- Unable to clear secretions – gagging
- Exercise limitations
- Unable to eat or drink as usual
Note: If child having severe breathing problems call 000 immediately and follow basic life support flowchart
- https://resus.org.au/guidelines/flowcharts-3/
Care of stoma site following discharge home
Ensure the caregivers are provided with adequate supplies and are aware of how to care for stoma site - this includes daily cleaning of the site and dressing changes as required. Advise the family/caregiver to contact the hospital and/or medical team if there are any signs of infection at the stoma site including any:
- Redness
- Odour
- Swelling
- Discharge
If stoma site remains open the family are advised to carefully supervise their child around water to avoid aspiration.
Documentation
Ensure all written documentation related to the management of a patient with a tracheostomy is in accordance with the RCH documentation policy.
Record the reason and type of the interventions performed relating to tracheostomy care and appropriate outcomes in the progress notes and flow sheets assessment.
These include:
- Suctioning (amount, colour and consistency of secretions)
- Tracheostomy cares performed including tie changes and stoma dressings
- Stoma condition (at least daily review and ongoing documentation and any changes e.g. signs of infection)
- When a tracheostomy tube change (routine or emergency) is performed document the date and time of the tracheostomy insertion, name of person who inserted the tube, size and type of tube inserted (including inner and outer diameter, tube length and suction depth), Lot number, expiry date of the tracheostomy tube, patient condition throughout and following the tube change and any difficulties experienced during or after the tracheostomy tube change.
Special Considerations
Should an aerosol generating procedure be undertaken on a patient under droplet precautions then increase to airborne precautions by donning N95/P2 mask for at least the duration of the procedure.
Companion Documents
- RCH PICU Guideline: Tracheostomy Tubes Cuff Management
Evidence table
Tracheostomy Management Evidence Table.
References
- Blackwood, and Bronagh. (1999). "Normal saline instillation with endotracheal suctioning: primum non nocere (first do no harm)" Journal of Advanced Nursing, 29 (4), 928-934.
- Carr, M. Poje, C.P. Kingston, L. Kielma, D. and Heard, C. (2001) "Complications in Pediatric Tracheostomies" Laryngoscope 111: November 2001.
- Celik, S. and Kanan, N (2006) " A current conflict use of Isotonic Sodium Chloride Solution on the Endotracheal Suctioning in Critically ill Patients" Dimensions of Critical Care Nursing vol 25/No1 pp:11-14.
- Choate, K and Snadford, M (2003) "Tracheostomy: Clinical Practice and the formation of policy and guidelines" Australian Nursing Journal, 10, 8 p: CU1.
- Dixon, L. and Wasson, D. (1998) "Comparing Use and Cost Effectiveness of Tracheostomy Tube Securing Devices. Medsurg Nursing, 7, 5 pp: 270-274
- Edwards, E.A. and Byrnes, C.A (1999) "Humidification Difficulties in Two Tracheostomized Children". Anaesthesia and Intensive Care, 27, 6, pp: 656-58.
- Evans, j., Syddall,S., Butt,W., and Kinney, S. (2014) "Comparison of open and closed suction on safety, efficacy and nursing time in a paediatric intensive care unit". Australian critical Care 27 (2014) 70 -74.
- Gray JE, MacIntyre NR, Kronenberger WG. The effects of bolus normal saline instillation in conjunction with endotracheal suctioning.
- Griggs, A. (1998) "Tracheostomy suctioning and humidification". Nursing Standard vol 13 (2) pp: 49-53, 55-56.
- Halm, M and Krisko-Hagel, K (2008) "Instilling Normal Saline with Suctioning: Beneficial Technique or Potentially Harmful Sacred Cow?" American Journal of Critical Care, 17: 469-472.
- Hussey, S.G, Ryan, C.A and Murphy, B.P. (2007) "Comparison of three manual ventilation devices using an intubated mannequin". Arch Dis. Child. Fetal Neonatal Ed. (2004); 89; 490-93.
- Oberwaldner, B. and Eber, E. (2006) "Tracheostomy care in the home". Paediatric Respiratory Reviews, 7, 185-190.
- O'Toole, EA. Wallis, C. (2004) "Sending children home on tracheostomy dependent ventilation:pitfalls and outcomes". BMJ vol 89 (3) pp: 251-255.
- Raymond SJ. Normal Saline Instillation Before suctioning: Helpful or Harmful? A review of the Literature". American Journal of Critical Care July 1995 Volume 4, No. 4 267-271.
- Respir. Care. 1990;35:785-790.
- Scoble M, Copnell, B. Taylor, A. Kinney, S and Shann, F. (2001) "Effect of reusing suction catheters on the occurrence of pneumonia in children" Heart and Lung vol 30, 3 p: 225-233.
- Schultz, J., Mitchell, M., Cooke, M., and Schibler, A. (2018) "Efficacy and safety of normal saline instillation and paediatric endotracheal suction: An integrative review". Australian Critical Care 31 (2018) 3-9.
- Ridling, D. Martin, LD and Bratton, S. (2003) "Endotracheal Suctioning With or Without Instillation of Isotonic Sodium Chloride Solution in Critically Ill Children". American Journal of Critical Care vol 12, no 3 pp:212-219.
- Tamburri, L.M. (2000) "Care of the Patient with a Tracheostomy". Orthopedic Nursing, 19, 2 pp:49-60.
- Wang, CH et al (2017) "Normal saline instillation before suctioning: A meta-analysis of randomized controlled trials". Australian Critical Care, Sep: 30(5): 260-265.
- Woodrow, P. (2002) "Managing patients with a tracheostomy in acute care". Nursing Standard vol 16 (44) pp: 39-48.
- Wyatt, M.E. Bailey, C.M. Whiteside, R.N (1999) "Update on paediatric tracheostomy tubes" The Journal of Laryngology and Otology , 113, 1, Health and Medical Complete pp:35-40.
- Wetmore, R.F. Marsh, R.R. Thompson, M.E. Tom, L.W. (1999) "Pediatric Tracheostomy: A Changing Procedure". The Annals pf Otology, Rhinology and Laryngology, 108, 7, pp:695 - 699.
Please remember to read the disclaimer.
The development of this nursing guideline was coordinated by Sueellen Jones, Registered Nurse, Respiratory Medicine, and approved by the Nursing Clinical Effectiveness Committee. Updated April 2018.
What Happens if a Trach Comes Out
Source: https://www.rch.org.au/rchcpg/hospital_clinical_guideline_index/Tracheostomy_management/